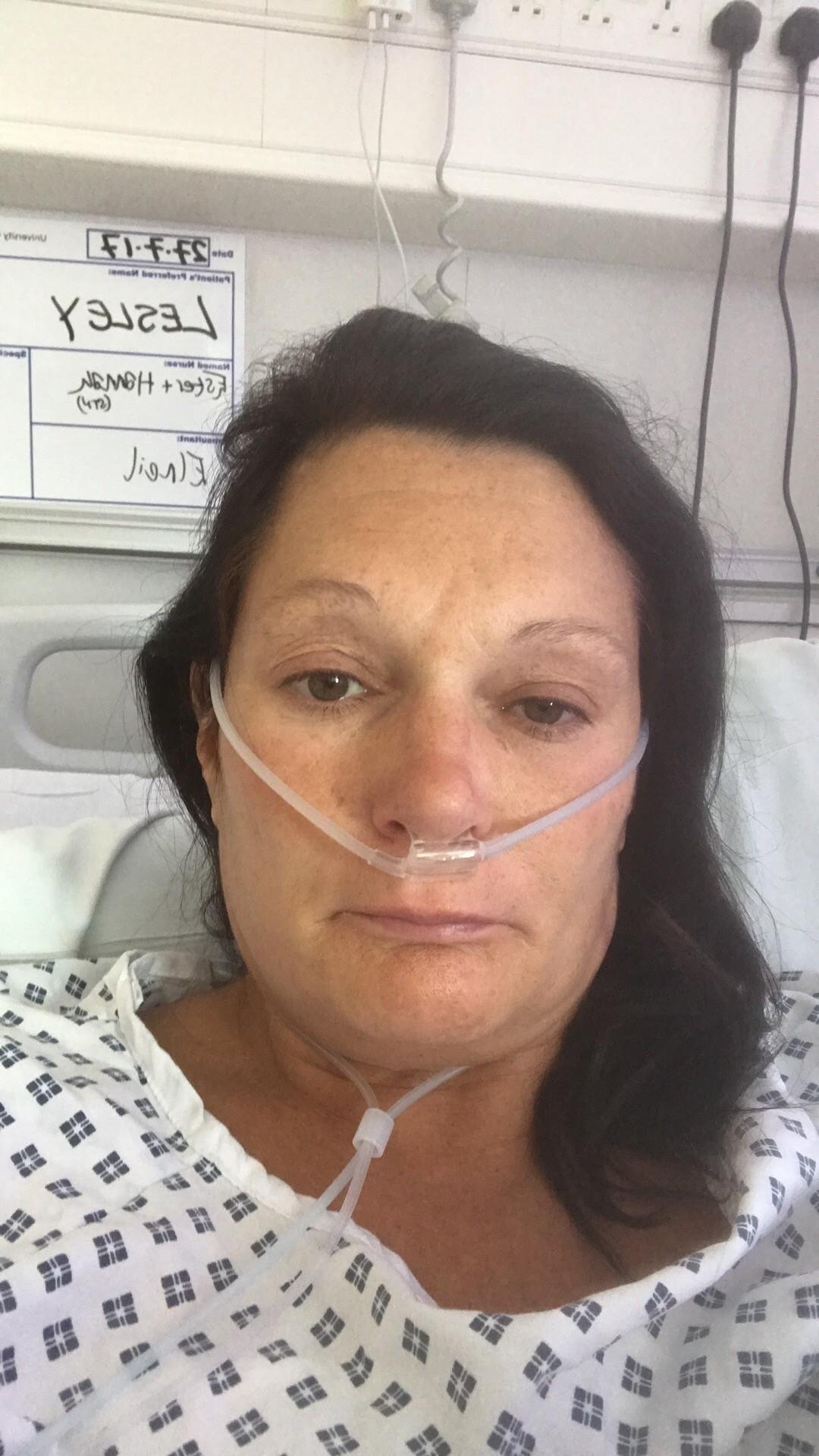
A MUM-OF-TWO has revealed she has been suicidal after a ‘barbaric’ vaginal mesh implant has left her unable to work, walk or have sex.
Lesley Elder, from Poole, hoped the routine gynaecology procedure would mean she could return to her active lifestyle.
However the implant has cut into her causing her chronic pain and is now embedded in her body.
The 50-year-old is now disabled, her marriage ended because she cannot have sex, she cannot work and is battling depression and post-traumatic stress disorder.
Lesley says she has endured 13 operations to try to end the pain to remove the mesh but like other women it is too embedded.
Vaginal mesh implants are offered to women with incontinence or prolapse after childbirth yet but hundreds are suing the NHS and the implants manufacturers after suffering crippling pain.
The plastic meshes are made of polypropylene - the same material used to make certain drinks bottles - and manufactured by many different companies.
Lesley, who has joined the Sling the Mesh campaign which is calling for the procedure branded by some medical professionals as ‘the biggest health scandal of our time’ to be suspended in the UK, said: “It’s been horrendous. I don’t feel like a woman anymore. I feel everything has gone out of my life.
“I’ve been back and forwards to hospital constantly.
“It is totally barbaric and people should know about it because this needs to be stopped.
“My life has not even been worth living anymore. It’s a nightmare it really is.”
Lesley explained before the controversial operation she enjoyed socialising, netball, running and going to the gym but had a type of mesh known as TOT fitted in December 2010 to cure her incontinence and her life turned upside down.
She explained: “The surgeon said it was a simple and fast operation to fix my bladder and that it would change my life.
“It certainly did change my life.
“I woke up in crucifying pain and I thought I was a man because that’s how swollen I was down there. I have never seen anything like it.
“I was in agony screaming and was rushed back into surgery bleeding. I had a haematoma. It was hell.”
Lesley discovered her implant had cut into her vagina causing her severe discomfort.
“My body is just wrecked. It feels like a barbed wire fence inside you. It’s been infection after infection after infection. I have to self-catheterise as it’s ruined all my bladder. Sometimes if I don’t do that I’m still wetting myself. ”
Lesley said the implant led to the breakdown of her marriage as she found intercourse too painful and her partner had even been injured by the mesh during sex.
“I tried a couple of years ago to have intercourse but it is just too painful so it’s been about seven years I’ve been without it but I have tried. I'm now single because nobody really wants to be in a relationship with someone who can't have sex.
"It’s just like glass down there. It’s like you sitting in your private area with broken glass with it burnt and you can’t shut your legs.
“Some days my mobility is fine but other days it really isn’t. It is a living nightmare.
“My dogs have been my life saviours.”
Today Lesley is recovering from her 13th operation at University College London Hospital with removal specialist Sohier Elneil.
Lesley, who has to walk aided with a stick, said: “It is still there. It’s stuck on my pubic bones at the moment.
"They couldn’t find the mesh because it is so embedded and it has also caused scar tissue so my bowels and bladder are stuck together. It’s like getting chewing gum out of your hair. It just doesn’t come out.
“It is a living nightmare. My life has been absolutely wrecked and I just want to get my life back and make others aware how dangerous this can be.”
A spokesman for Medicines and Healthcare products Regulatory Agency said: "Patient safety is our highest priority and we sympathise with women who have suffered complications after surgery.
“We are committed to helping address the serious concerns raised by some patients. We have undertaken work to assess the findings of studies undertaken by the clinical community over many years, as well as considering the feedback from all sources in that time.
“What we continue to see is that evidence supports the use of these devices in the UK for treatment of the distressing conditions of incontinence and organ prolapse in appropriate circumstances. This is supported by the greater proportion of the clinical community and patients.
“In common with other medical device regulators worldwide, none of whom have removed these devices from the market, we are not aware of a robust body of evidence which would lead to the conclusion these devices are unsafe if used as intended.
“We actively encourage patients and healthcare professionals to report complications associated with these implants through the Yellow Card scheme, or via gov.uk/report-problem-medicine-medical-device.”









Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel